DIASAFE®plus dialysis fluid filter

A key step towards good dialysis practice
- Produces ultrapure dialysis fluid
- Provides sterile and non-pyrogenic substitution fluid*
* When used with two DIASAFE®plus in series (for ONLINE therapies)
Key features
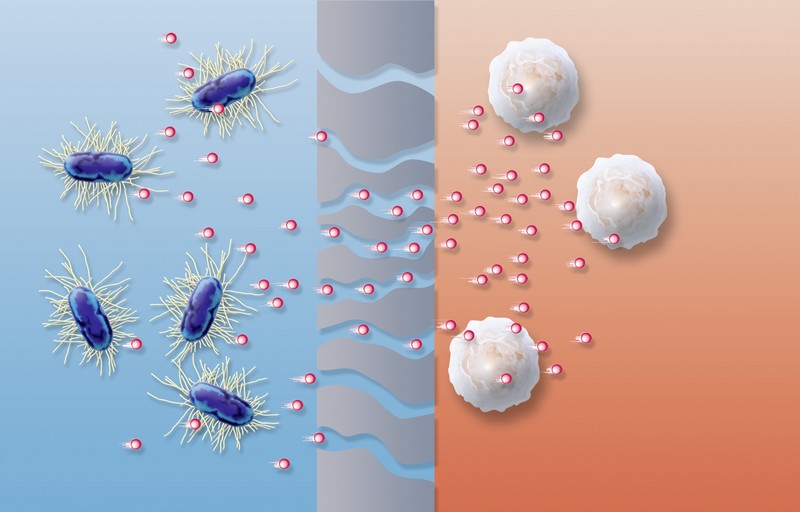
Figure 1: Fragments of bacterial endotoxins enter the patient’s bloodstream and activate leukocytes, therefore leading to acute and chronic complications in haemodialysis patients.
Dialysis fluid purity
The quality and purity of dialysis fluids are of major concern in modern renal replacement therapies, as large volumes of dialysis fluid come into contact with the patient’s bloodstream. Dialysis fluids may contain microbial impurities, such as endotoxins derived from bacterial fragments. Endotoxin fragments may have molecular weights well below 2000Da - small enough to pass across both low and high flux membranes (Figure 1). Endotoxins are known to cause acute adverse reactions and promote long-term complications in haemodialysis patients1,2.
In order to avoid endotoxin-related complications during routine hemodialysis, the European Renal Best Practice Guidelines for Haemodialysis (EBPG)3 strongly recommend the usage of ultrapure water. Ultrapure water or dialysis fluid can easily be achieved through the application of special dialysis fluid filters, such as DIASAFE®plus.
| The different purity levels of pure and ultrapure water according to the European Renal Best Practice Guidelines for Haemodialysis | ||
|---|---|---|
| Pure water | Ultrapure water | |
| Microbial contaminations (CFU/ml) | ≤ 100 | < 0.1 |
| Bacterial endotoxins (IU/ml) | < 0.25 | < 0.03 |
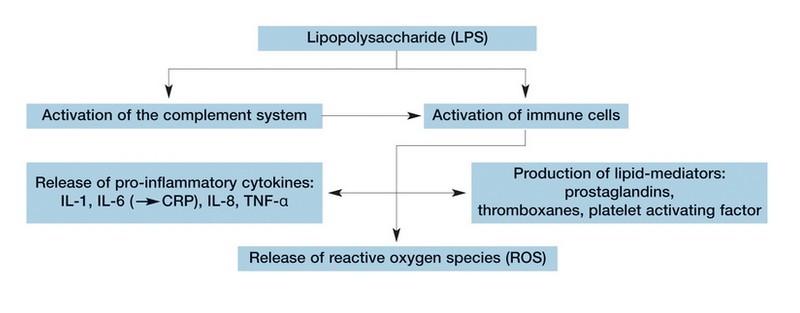
Endotoxins (LPS) stimulate the release of pro-inflammatory cytokines, reactive oxygen species and lipid mediators from immune-competent cells.
Chronic inflammation
Endotoxins can activate immune component cells in a number of ways, contributing to chronic inflammation that is present in all haemodialysis patients4. Evidence demonstrates that chronic inflammation is a major risk factor for progressive atherosclerotic Cardiovascular Disease (CVD)5.
The usage of ultrapure dialysis fluid has been shown to reduce markers of chronic inflammation in haemodialysis patients6; therefore, it is suggestive that ultrapure dialysis fluid has a beneficial effect on inflammatory diseases such as atherosclerotic CVD7.
Oxidative stress
Several treatment related stimuli, including bacterial endotoxins derived from dialysis fluid8,9 can increase oxidative stress, a situation in which the normal balance between production of Reactive Oxygen Species (ROS) and antioxidant activity is tilted in favour of ROS. As oxidative stress is associated with the progression of malnutrition, anaemia and inflammatory diseases such as atherosclerosis, the usage of ultrapure dialysis fluid to reduce dialysis induced oxidative mechanisms appears desirable8,9.
Advanced Glycation End Products (AGE)
The importance of ultrapure dialysis fluid in routine haemodialysis treatments is emphasised by the finding that endotoxins act in synergy with AGE, which enhance inflammation and oxidative stress10. The use of ultrapure dialysis fluid has further been shown to reduce the plasma levels of the AGE compound pentosidine11.
Anemia treatment
Ultrapure dialysis fluid has been shown to improve iron utilisation and the response to erythropoietin, meaning it could be beneficial in anaemia treatment by allowing for a reduced erythropoietin dosage while maintaining optimal haemoglobin levels12,13.
Technology



DIASAFE®plus : a key step towards good dialysis practice
Although water used for the production of dialysis fluid is treated by a series of purification steps, it still may not meet the stringent requirements on bacterial contamination levels laid down by regulatory bodies. Located at the end of the water treatment chain, the DIASAFE®plus filter ensures required purity levels can be achieved easily through the reliable production of ultrapure dialysis fluid to ISO standards. The DIASAFE®plus incorporates the Fresenius Polysulfone® membrane, which has excellent endotoxin retention capabilities due to the superior adsorptive and sieving characteristics of the membrane. The extended surface area of 2.2 m² further extends the adsorption and retention capacity.
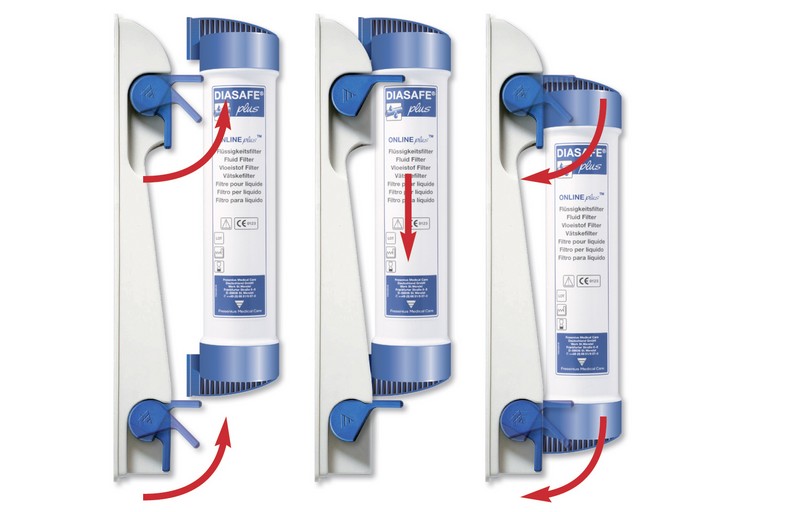
DIASAFE®plus can be connected with only three rapid handling steps. The DIAFIX™ lock system provides an easy and hygienic connection.
Hygienic, with fast and easy installation and exchange
The DIAFIX™ lock system provides an easy and hygienic connection of the DIASAFE®plus to the dialysis machine with only three handling steps necessary for installation or exchange:
- Open the locks of the filter holder
- Slide the DIASAFE®plus filter into the guide grooves
- Close the locks. DIASAFE®plus is ready to use. It’s that easy!
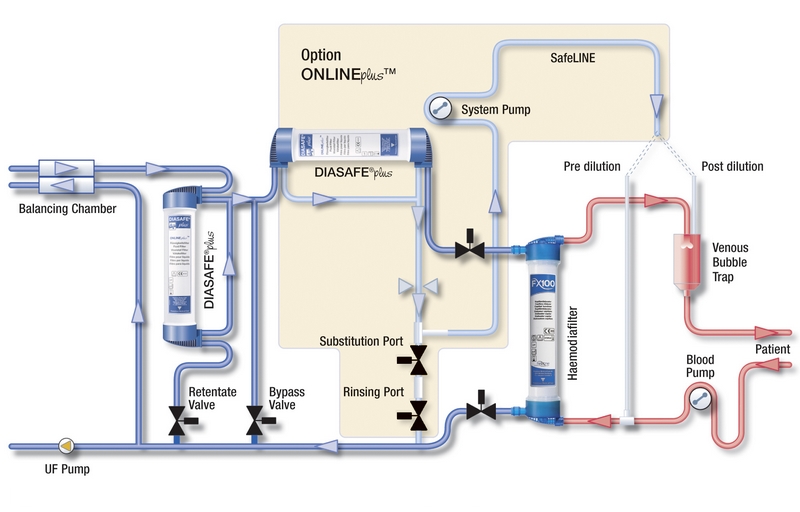
DIASAFE®plus in the ONLINEplus™system
- Ultrapure dialysis fluid prepared with DIASAFE®plus, together with haemodialysers containing endotoxin-retaining membranes (Fresenius Polysulfone®, Helixone® or Helixone®plus) are the main building blocks for a high-quality haemodialysis treatment
- The ONLINEplus™ system takes the quality standards of convective treatment modalities such as Haemodiafiltration/Haemofiltration (HDF/HF) one step further
- Reliable production of sterile and non-pyrogenic substitution fluid is achieved by double filtration for ONLINE HDF/HF therapies, when using two DIASAFE®plus fluid filters in series14
- Besides improving hygiene and safety of convective therapy modalities, the ONLINEplus™ option also offers additional treatment features and easier handling
Performance data
DIASAFE®plus dialysis fluid filters
| DIASAFE®plus dialysis fluid filters | ||||||
|---|---|---|---|---|---|---|
| Membrane material | Fresenius Polysulfone® | |||||
| Effective surface (m²) | 2,2 | |||||
| Weight (g) | 170 | |||||
| Housing material | Polypropylene | |||||
| Potting material | Polyurethane | |||||
| Sealings | Silicone | |||||
| Connection to machine | DIAFIX™ Lock System | |||||
| Filtration rate | 5 ml/min x mm Hg (3.75 l/min bar; max. 2 bar) | |||||
| Operating time | Standard HD: max. 12 weeks or 100 ONLINE HF/HDF treatments; ONLINE priming/rinsing: max. 12 weeks or 100 treatments | |||||
| Disinfection | Puristeril® 340 or Puristeril®plus (peracetic acid) Diasteril® (hydroxyacetic acid) or Citrosteril® (citric acid) Sporotal®100 (sodium hypochlorite) max. 11 times | |||||
If you would like to order this product via the NHS Supply Chain Catalogue, please visit the following link: NHS Supply Chain Online Catalogue
Additional information relating to multiBic or Calrecia can be found in the critical care section of our product information page.
Adverse Events Reporting
Adverse events should be reported. Reporting forms and information can be found at
https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple app store. Adverse events should also be reported to Fresenius Medical Care on 01623 445100.
UK/HEMA/FME/0922/0005 Date of Preparation: November 2022
1 Dasgupta MK: Biofilms and infection in dialysis patients. Seminars in Dialysis 15: 338-346, 2002.
2 Brunet P and Berland Y: Water quality and complications of haemodialysis. Nephrology Dialysis Transplantation 15: 578-580, 2000.
3 European Best Practice Guidelines for Haemodialysis (Part 1), Section IV – Dialysis fluid purity. Nephrology Dialysis Transplantation 17 (Suppl. 7): 45-62, 2002.
4 Ward DM: Hemodialysis water: an update on safety issues, monitoring and adverse clinical effects. ASAIO American Society for Artificial Internal Organs 50 (6): XIII-XIX, 2004.
5 Yao Q et al.: Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease and poor outcome in hemodialysis patients. Hemodialysis International 8: 118-129, 2004.
6 Schiffl H et al.: Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrology Dialysis Transplantation 16: 1863-1869, 2001.
7 Lonnemann G: When good water goes bad: how it happens, clinical consequences and possible solutions. Blood Purification 22: 124-129, 2004.
8. Locatelli F et al.: Oxidative stress in end-stage renal disease: an emerging threat to patient outcome (consensus paper). Nephrology Dialysis Transplantation 18: 1272-1280, 2003.
9 Ward RA and McLeish KR: Oxidant stress in hemodialysis patients: what are the determining factors? Artificial Organs 27: 230-236, 2003.
10 Reznikov LL et al.: Effect of advanced glycation end products on endotoxin-induced TNF-a, IL-1 and IL-8 in human peripheral blood mononuclear cells. Clinical Nephrology 61: 324-336, 2004.
11 Izuhara Y et al.: Ultrapure dialysate decreases plasma pentosidine, a marker of carbonyle stress. American Journal of Kidney Diseases 43: 1024-1029, 2004.
12 Sitter T et al.: Dialysate related cytokine induction and response to recombinant human erythropoietin in hemodialysis patients. Nephrology Dialysis Transplantation 15: 1207-1211, 2000.
13 Hsu PY et al.: Ultrapure dialysate improves iron utilisation and erythropoietin response in chronic haemodialysis patients – a prospective cross-over study. Journal of Nephrology 17: 693-700, 2004.
14 Weber C et al.: Novel online infusate-assisted dialysis system performs microbiologically safely. Artificial Organs 24: 323-328, 2000.