communication Data Link (cDL)

Interfacing the TDMS with external Hospital Information Systems (HIS)
- Consistent use of patient data
- Supports billing processes
- Enables treatment documentation in electronic health records

At a glance
- Designed with you in mind, for seamless Therapy Data Management System (TDMS) interconnection with an existing Hospital Information System (HIS).
- Efficient management of patient and treatment data starts with the correct information being available in the right place at the right time.
- The primary function of cDL is to facilitate and process bidirectional communications between the Therapy Manager and the HIS, ensuring quality and efficiency.
- Developed exclusively for application in a medical environment; no additional set-up programming is required.
Advantages for administrators
Fresenius Medical Care middleware provides a number of advantages for clerical staff
- Resource optimisation
- Data protection
- Transparency
- Flexibility
- Product simplicity
Advantages for clinical staff
Improving the experience of patients and clinical staff alike
- Optimised treatment
- Optimised patient care
In Depth
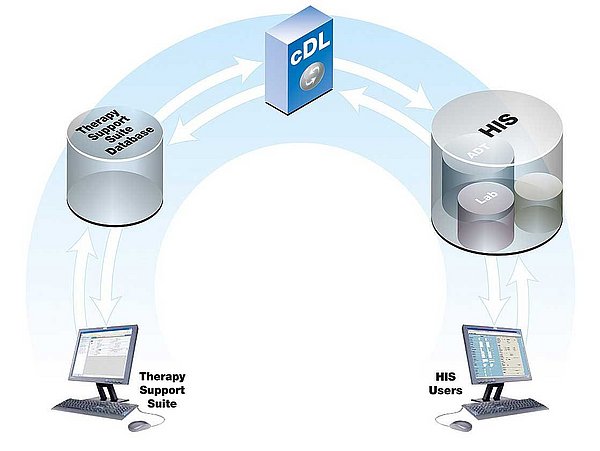
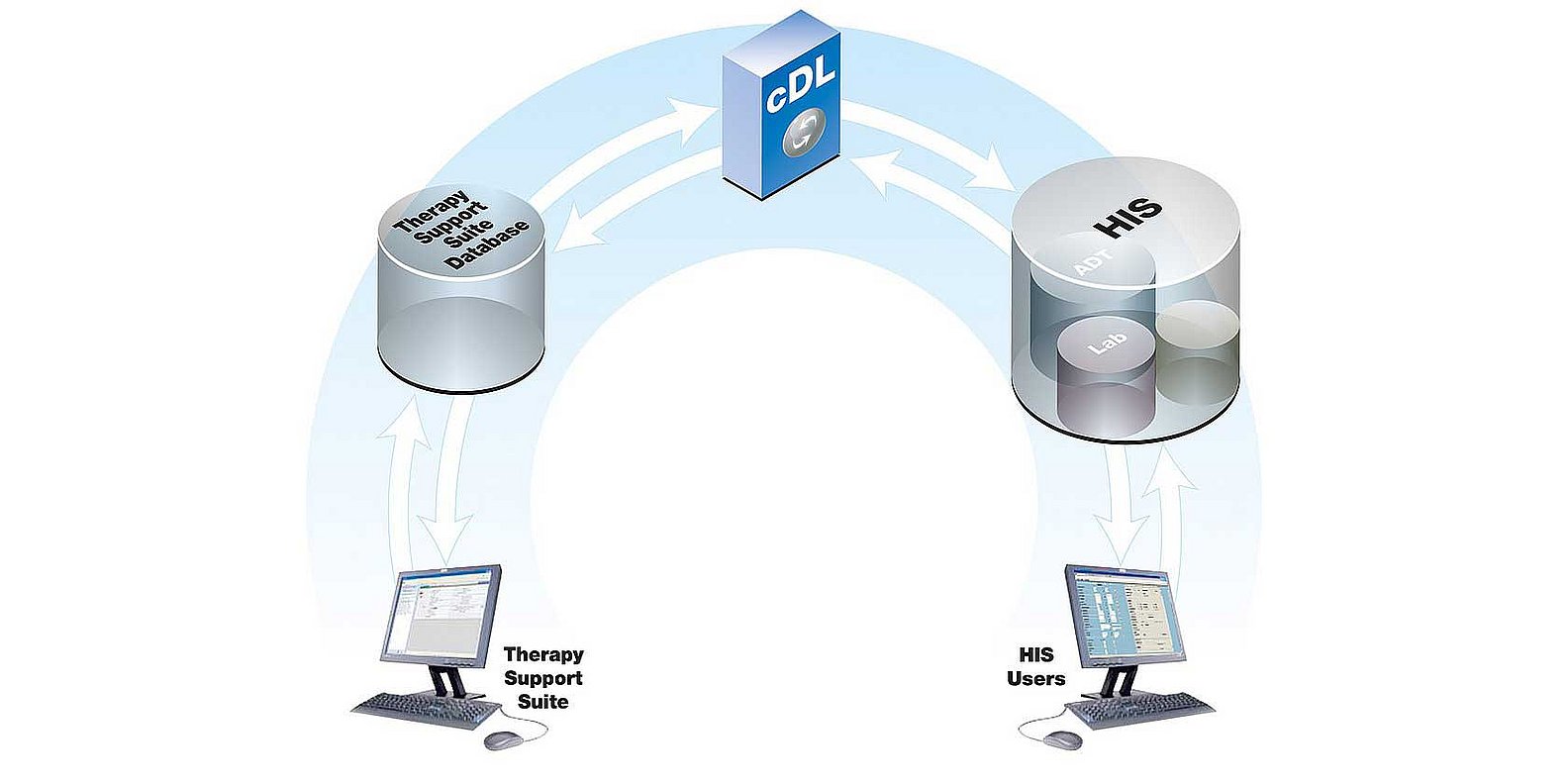
| Resource optimisation | Reduces administration by eliminating the need for data to be stored in multiple databases. |
|---|---|
| Data protection | Data is only stored in the secure TDMS database and HIS. |
| Transparency | Relevant data is available to all users at their respective interfaces. |
| Flexibility | Uses standard HIS protocols, but can be configured to comply with local systems. |
| Product simplicity | Minimal maintenance and no custom installation required. |
| Optimised treatment | Improved availability of comprehensive patient data and lab results. |
| Optimised patient care | Reduced administration allows more time for patient care. |
Additional information relating to multiBic or Calrecia can be found in the critical care section of our product information page.
Adverse Events Reporting
Adverse events should be reported. Reporting forms and information can be found at
https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple app store. Adverse events should also be reported to Fresenius Medical Care on 01623 445100.
UK/HEMA/FME/0922/0005 Date of Preparation: November 2022

